Access the full NECO Chemistry Answers for 2025 here. This page will be updated with accurate solutions to both objective and essay questions before the exam starts. Refresh the page regularly to see the latest answers.
NECO Chemistry OBJ Answers 2025
01-10: CEDADDCCEC
11-20: ECBAEBAEBD
21-30: BCBDEABCAE
31-40: CDDCBBCDED
41-50: DAEAADDAED
51-60: DBCEECDDBE
COMPLETED!!!
NECO Chemistry Essay Answers 2025
Number 1
(1ai)
Fermentation is a biochemical process in which glucose (or other sugars) is broken down into ethanol and carbon dioxide by yeast or bacteria under anaerobic conditions.
(1aii)
Ethanol Production:
2(C₆H₁₀O₅)n(s) + nH₂O(l) —> nC₁₂H₂₂O₆(aq)
C₁₂H₂₂O₁₁(aq) + H₂O(l) —> 2C₆H₁₂O₆(aq)
C₆H₁₂O₆(aq) —> 2C₂H₅OH(aq) + 2CO₂(g)
(1b)
[FILL IN THE TABLE]
=UNDER FLOURINE=
State at room temperature: Gas
=UNDER FLOURINE=
State at room temperature: Gas
(1ci)
MgCO₃:
Mg = 24, C = 12, O = 16
Molar mass of MgCO₃ = 24 + 12 + (3 × 16) = 24 + 12 + 48 = 84 g/mol
Number of moles= Mass/Molar mass
Number of moles = 25/84 = 0.2976 mol
(1cii)
Methyl orange
(1ciii)
I. Acidic oxide: Carbon dioxide (CO₂)
II. Neutral oxide: Nitrous oxide (N₂O)
(1civ)
Hydrophobic compounds repel water, while hydrophilic compounds attract water.
(1cv)
I. CH₃CH₂COOH: Propanoic acid
II. CH₃CH₂CH₂CH₂OH: Butan-1-ol
(1di)
The rate of a chemical reaction is the change in the concentration of reactants or products per unit time.
(1dii)
(i) Concentration of reactants
(ii) Temperature
(iii) Presence of a catalyst
==================
Number 2
(2ai)
An acid anhydride is a non-metallic oxide that reacts with water to form an acid.
(2aii)
(i) Ethanoic acid (CH₃COOH)
(ii) Carbonic acid (H₂CO₃)
(2aiii)
Fats are solids at room temperature, while oils are liquids at room temperature.
(2b)
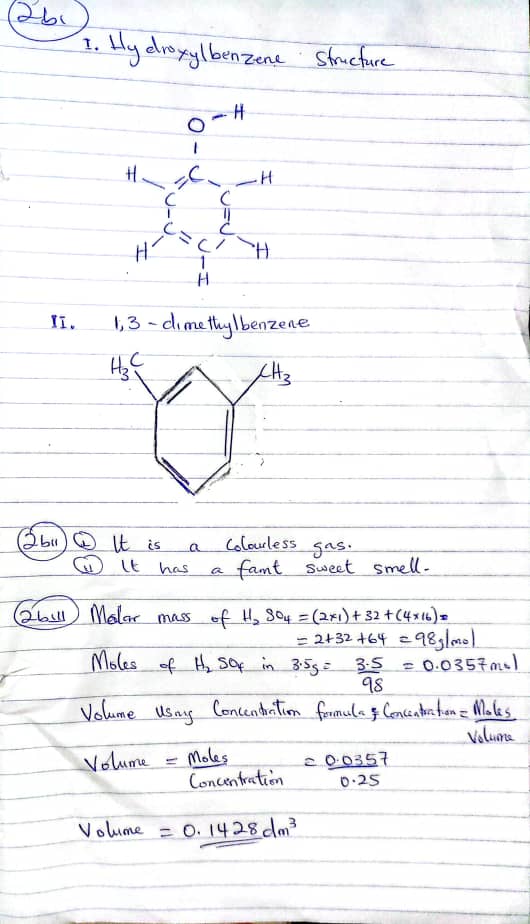
(2ci)
The periodic law states that the properties of elements are a periodic function of their atomic numbers.
(2cii)
[TABULATE PLEASE]
=Beryllium=
Atomic Number: 4
Electronic Configuration: 1s² 2s²
Valence Electrons: 2
=Aluminium=
Atomic Number: 13
Electronic Configuration: 1s² 2s² 2p⁶ 3s² 3p¹
Valence Electrons: 3
=Potassium=
Atomic Number: 19
Electronic Configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
Valence Electrons: 1
(2ciii)
I. Highest Electronegativity: ₁₇D
II. Same Group: ₃A and ₁₁B
(2civ)
Lamp black
==================
Number 3
(3ai)
Entropy is defined as a measure of the degree of disorder or randomness in a system. It quantifies how energy is distributed among the particles in a system and how much of that energy is unavailable to do useful work.
(3aii)
Given:
Volume of O₂ = 0.28dm³ = 280cm³
Mass of O₂ = 0.4g
Mass of 1000 cm³ (1 dm³) of H₂ = 0.090g
Molar mass of hydrogen = 2g/mol
Vapour density = (mass of certain volume of gas)/ (mass of same volume of hydrogen) ×(1/2)
Mass of 280cm³ of H₂ = (0.090/1000) × 280 cm³ = 0.0252g
Vapour density = 0.4/0.0252 = 15.87
(3bi)
I. Increase the pressure
II. Increasing pressure favors the side with fewer moles of gas, shifting the equilibrium toward the product side if it has fewer moles, or lowering temperature favors exothermic forward reaction, increasing product yield.
(3bii)
I. Tetraoxosulphate (VI) acid:
-Fertilizer production
-Petroleum refining
II. Diamond:
-Jewelry
-Cutting tools
III. Carbon (IV) oxide:
-Fire extinguisher
-Photosynthesis
(3biii)
(i) Sodium chloride (NaCl)
(ii) Potassium nitrate (KNO₃)
(3ci)
(i) Colorless liquid
(ii) Aromatic odor
(3cii)
3,4-dimethylpentan-2-ol
(3ciii)
(i) Store chemicals in cool, dry places.
(ii) Use airtight containers.
(3civ)
Graphite
(3di)
Basicity of Ethanoic Acid is 1
(3dii)
(i) Strong electrolyte: Sodium chloride (NaCl)
(ii) Weak electrolyte: Acetic acid (CH₃COOH)
==================
Number 4
(4ai)
Reforming is the process of changing the structure of hydrocarbon molecules to produce better fuels like petrol.
(4aii)
Catalytic reforming
(4aiii)
(i) It helps to produce more petrol.
(ii) It breaks large molecules into smaller, more useful ones.
(4bi)
I. Sodium: Yellow flame
II. Calcium: Brick red flame
(4bii)
[Ca = 40]
Mass = number of moles × molar mass
= 0.50 × 40 = 20g
(4biii)
Molar mass of nitrogen gas (N₂) = 14 × 2 = 28 g
Number of moles = mass / molar mass = 14 / 28 = 0.5 moles
(4ci)
I. Charles’ law: Pressure
II. Graham’s law of diffusion: Temperature and pressure
(4cii)
I. Chalk dust in water: Filtration
II. Petroleum: Fractional distillation
(4ciii)
Petroleum industry
(4civ)
I. Fuel for jet engines: Kerosene
II. Fuel for heavy duty machines: Diesel
III. Coating agent in pipes: Bitumen
(4di)
Calcium oxide (CaO)
(4dii)
Formula: XY₂
(4diii)
(i) Thermoplastics
(ii) Thermosetting plastics
==================
Number 5
(5ai)
(i)It is a colorless gas.
(ii)It has a sweet odor.
(5aii)
(i)They exhibit variable oxidation states.
(ii)They form colored compounds.
(iii)They act as catalysts.
(5aiii)
(i)Coagulation/Flocculation: Adding chemicals like alum to make suspended particles clump together.
(ii)Filtration: Passing water through layers of sand and gravel to remove larger impurities.
(5bi)
I. Current (I) = 0.5 A
Charge (Q) = 9650 C
Faraday constant (F) = 96500 C/mol
Molar mass of Cu = 64 g/mol
n (valency of Cu²⁺) = 2
Mass (m) = (Q × M) / (n × F)
= (9650 × 64) / (2 × 96500)
= 617600 / 193000
= 3.2 g
Mass of copper deposited = 3.2g
II. Use the formula Charge = Current × Time.
Time Charge/current 9650C/0.5A
=19300 seconds.
(5bii)
Electrolytic refining
(5biii)
(i)Limestone (calcium carbonate)
(ii)Clay (silica, alumina, iron oxides)
(5biv)
Used in construction to bind materials together, forming concrete and mortar.
(5ci)
Denaturation and hydrolysis of the protein occur.
(5cii)
(i)Amino group (-NH2)
(ii)Carboxyl group (-COOH)
(5ciii)
I. Hydrogen gas (H2).
II. Water vapor (H₂O).
III. It is a colorless and odorless gas.
(5civ)
[Mn] + [-2×2]=0
[M]-4=0
[mm] = +4
oxidation state of Manganese in MnO2 = +4
==================
Number 6
(6ai)
An isotope is one of two or more atoms of the same element that have the same number of protons but different numbers of neutrons.
(6aii)
I. Given isotopes: ³³₁₅Z and ³¹₁₅Z in a 1:3 ratio
Relative atomic mass = ((33 × 1) + (31 × 3)) / (1 + 3)
= (33 + 93) / 4
= 126 / 4
= 31.5
II. The relative atomic mass is not a whole number because it’s a weighted average of the isotopic masses, considering their abundance.
(6bi)
Because there is a strong force of attraction between the positive and negative ions.
(6bii)
(i) Water (H₂O)
(ii) Ammonia (NH₃)
(6biii)
(i) They dissolve easily in water.
(ii) They conduct electricity when molten or dissolved in water.
(6ci)
4Fe + 3O₂ + 6H₂O —> 4Fe(OH)₃
(6cii)
(i) Carbon
(ii) Silicon
(6ciii)
Luminous flame is yellow and sooty while non-luminous flame is blue and clean.
(6di)
I. A black precipitate of lead(II) sulphide is formed.
II. The orange solution turns green.
(6dii)
It is a colourless gas with a rotten egg smell.
(6ei)
CO₂ + 2NaOH —> Na₂CO₃ + H₂O
(6eii)
(i) Air pollution
(ii) Water pollution
(iii) Soil contamination
==================
COMPLETED!!!
